The Geological Fingerprint of War
March 16, 2012

Earle McBride and Dane Picard were traveling across France doing geologic field work in 1988 when they took time out to play tourists at Omaha Beach, site of one of the most ferocious battles during the D-Day invasion more than 40 years earlier. It was a miserably cold and blustery day. They tarried just long enough to scoop a sample of beach sand into a little baggie.
McBride, a professor emeritus in the Jackson School of Geosciences at The University of Texas at Austin, collects sand pretty much any chance he gets. By analyzing sand from modern dunes, beaches and rivers from a wide range of sites around the world, he can link the mineral compositions of ancient sandstones to the kinds of environments that forged them.
A few years after the French trip, he put the beach sand under a microscope and discovered tiny metal shards mixed in with the ordinary bits of quartz and other materials that he expected to see. Those shards turned out to be shrapnel from the famous World War II invasion. On closer examination, he also found iron and glass beads that resulted from the intense heat unleashed by explosions in the air and sand
“It is of course not surprising that shrapnel was added to the Omaha Beach sand at the time of the battle, but it is surprising that it survived 40-plus years and is doubtless still there today,” wrote McBride and Picard, currently a professor emeritus at the University of Utah, in an article for Earth Magazine last year.

In the early hours of June 6, 1944, more than 160,000 Allied troops poured from planes and ships onto the heavily fortified shores of Normandy, France. Omaha Beach was one of five Allied landing points along an 80-kilometer stretch of coastline.
“The battles were bloody and brutal,” wrote McBride and Picard, “but by day’s end, the Allies had established a beachhead.”
It proved to be the turning point of the war. McBride was just 12 years old in 1944.
“We’d hear daily reports on the progress of the war in Europe,” he said. “It was all so far away. I knew where France was, but I didn’t know where Normandy was.”
To analyze the sand, McBride first mixed the tiny grains with a blue epoxy, making what amounted to artificial sandstone, and then sliced it into thin sections. Under an optical microscope operating in transmission mode (in which light passes through the sample), he could see opaque grains. Adding another light source to see reflected light, the grains appeared shiny, an unusual feature for naturally occurring minerals. The shard-like angularity of the grains suggested these were not naturally formed. Ordinary wave action tends to blunt sharp edges. Other tests showed the metal shards contained large amounts of iron and were magnetic. At this point, he had no doubt these were pieces of shrapnel.
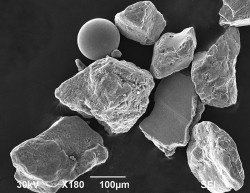
McBride reported that 4 percent of the sand is made up of these bits of shrapnel ranging in size from very fine to course (0.06 to 1 millimeter). Because the beach surface is continually being reworked by wind and waves, a sample taken on another day might have yielded a different abundance. He also found trace amounts of spherical iron beads and glass beads. Some iron beads were broken, revealing hollow centers. Using a scanning electron microscope, he was able to study the shape, texture, and size of all three explosively-produced structure types in greater detail.
McBride and Picard published their full results in the September 2011 edition of The Sedimentary Record, a quarterly journal of The Society for Sedimentary Geology (SEPM).
“Today, the only visible indications of the horrific battles fought at Omaha Beach are some concrete casements above the beach and nearby cemeteries that quietly mark the thousands of lives lost,” wrote McBride and Picard.
Gone are the wrecks of planes, ships and tanks, the shell casings, the scraps of rotted boot leather, and all the other detritus of war long since spirited away by generations of beachcombers. And so it fell to a pair of geologists to pluck one last relic from the sand, hidden under the feet of thousands of tourists every year.
Unlike the global layer of radioactive fallout from the 1950s atomic bomb tests that geologists and others now use to calibrate their tools for dating geologic materials, the microscopic fingerprint of the D-day invasion probably won’t endure long.

McBride says the iron-rich shrapnel shards could probably withstand the scouring action of waves alone for hundreds of thousands of years. But studying the shrapnel grains under high magnification, he observed particles of iron oxide, or rust, created by a chemical reaction between saltwater and iron. Waves churn the iron fragments, which rubs off some of the rust and exposes fresh material, which is more amenable to rusting, which in turn gets rubbed off, and so on.
“The net result is these things will get smaller and smaller and then finally get carried away by storms or hurricanes and be taken out of the beach,” says McBride. “So their time is numbered.”
“[T]he combination of chemical corrosion and abrasion will likely destroy the grains in a century or so,” wrote McBride and Picard, “leaving only the memorials and people’s memories to recall the extent of devastation suffered by those directly engaged in World War II.”
by Marc Airhart
Also See:
Video: “Europe Invaded,” Fox Movietone News, Jun. 13, 1944
This classic newsreel highlights the Invasion of Rome and D-Day Invasion.
<><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><>
SIDEBAR: Sand and Forensics

In the 1960s, detectives with the Texas Department of Public Safety brought Earle McBride a sample of sand collected from the pant cuff of a murder suspect. They wanted to know if the suspect had been to the Rio Grande.
Within seconds, McBride could tell that the sand was from the Colorado River near Austin. Some telltale signs: It had pink potassium feldspar grains derived from granite in the Llano region, which are commonly found in the Colorado River but not in the Rio Grande; and there were no sand grains derived from volcanic rocks, something common in sands from the Rio Grande but not from the Colorado.
“Unfortunately, that wasn’t the answer the police wanted, so I got dismissed,” he said. “That was my first foray into forensic science.”
McBride’s sand collection is carefully stored in hundreds of bags and bottles in row after row of metal drawers in the basement of the Jackson Geosciences Building.
Once, three agents from the FBI’s anti-terrorism unit paid him a visit to collect about a hundred pea-sized samples from locations around the world.
“They wanted to see if they could devise some new black box analytical technique so that if they got sand from the bottom of a shoe of a suspect, could they tell where the suspect came from?” he said.
It all sounds like something out of the TV show CSI. McBride acknowledges there are limitations to what you can learn from a single sample of sand.
“There are some fairly humorous examples I’ve seen on CSI where they get a little sample from a tire and they say, ‘Oh yeah, I know where that came from. That’s from 42nd Street and 12th Avenue,'” he deadpans. “It doesn’t work that way.”
<><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><><>