Forever Chemical Pollution Can Now Be Tracked
August 7, 2024

Organofluorine compounds — sometimes called ‘forever chemicals’ — are increasingly turning up in our drinking water, oceans and even human blood, posing a potential threat to the environment and human health.
Now, researchers at The University of Texas at Austin have developed a way to fingerprint them, which could help authorities trace them to their source when they end up in aquifers, waterways or soil.
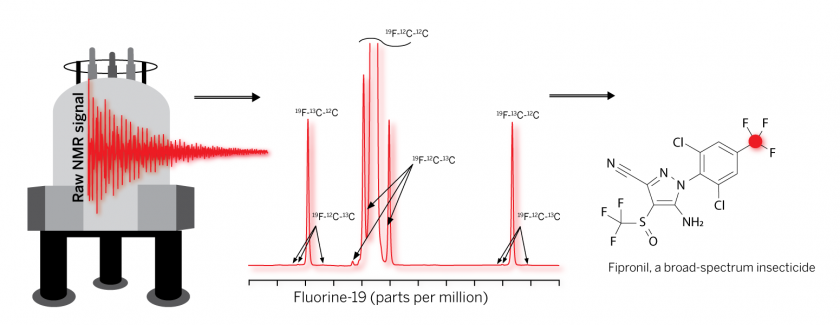
The technique involves passing samples through a strong magnetic field then reading the burst of radio waves their atoms emit. This reveals the composition of carbon isotopes in the molecule and gives the chemical its fingerprint, a feat that had not previously been achieved with forever chemicals.
The work is important because it allows scientists to track the spread of forever chemicals in the environment, said Cornelia Rasmussen, a research assistant professor at the University of Texas Institute for Geophysics at the Jackson School of Geosciences.
“Ultimately we will be able to trace molecules and see how they move,” said Rasmussen, who co-led development of the technique. “For example, whether they just stay where they got dumped or whether they’re moving downstream.”
The new technique was described in a paper published in the journal Environmental Science & Technology.
The super strong molecular bonds that give forever chemicals their handy characteristics — which are put to use in everything from fire retardants to non-stick surfaces and slow-release drugs — also keep them from breaking down in the environment, causing them to build up as pollution in soil and organic material to which they easily stick.
The U.S. Environmental Protection Agency plans to regulate forever chemicals, which include PFAS, and eliminate most of them from drinking water. However, the molecular bonds of the chemicals also make them difficult to trace. That’s because conventional chemical fingerprinting involves breaking molecules apart in a mass spectrometer which doesn’t work well with the tough molecular bonds of forever chemicals.
Instead, the researchers turned to a technology called nuclear magnetic resonance (NMR) spectroscopy, which measures a molecule’s structure and identifies its isotopes without breaking it apart.
Isotopes refer to chemical elements with differences in the number of neutrons in its atoms. Forever chemicals are made by bonding carbon isotopes to the element fluorine, which almost never happens in nature. Once the molecular bonds form, they are virtually unbreakable.
The researchers’ technique uses the NMR instrument alongside their own computational tools to determine the mix of carbon isotopes at each position in the molecule. Because the mix of carbon isotopes bonding to each fluorine atom is unique to how the chemical was manufactured, this information can be used like a fingerprint to trace a chemical.
It’s like a built-in barcode for molecules, said coauthor, David Hoffman, an associate professor at the Department of Molecular Biosciences in UT’s College of Natural Sciences.
“Part of the reason this has worked out so well is because we’re assembling tools from different areas of science [chemistry and geosciences] that don’t normally mix and using them to do something no one’s really done before,” he said.
The researchers tested their technique on samples that included pharmaceuticals and a common pesticide. Rasmussen and Hoffman are now conducting a pilot study to see how the technique will fare on pollutants that show up in the city of Austin’s creeks and wastewater. If successful, the technique could be useful for state and federal agencies who want to track the spread of water-borne forever chemicals.
Rasmussen said that the work has opened up a new layer of isotope information in organic chemistry that could find many applications beyond tracking forever chemicals, such as detecting counterfeit drugs or astrobiology. Her ultimate goal, however, is to take the technique even further afield.
“It’s given us a whole range of possibilities to learn really interesting things about metabolism on early Earth,” she said. “It could even tell us whether organics on Mars are the last remnants of some ancient Martian life.”
The research was funded by the U.S. Department of Energy’s Basic Energy Sciences program.
For more information, contact: Anton Caputo, Jackson School of Geosciences, 512-232-9623; Monica Kortsha, Jackson School of Geosciences, 512-471-2241; Constantino Panagopulos, University of Texas Institute for Geophysics.