Discussion
Potassium Hydroxide Electrolyte
Fe-purified vs Fe-containing Electrolyte
Before potential cycling, we evaluated the KOH electrolyte for the presence of metals that have the possibility of improving the HER. This will enable us to have a better understanding of the possible contributions of these metallic impurities to the HER activity and provide insight into how to better evaluate the electrochemical performance of catalysts. After the ICPMS analysis, as shown in Table 1, the unpurified electrolyte contains Fe, Ag, and Pb. This is quite insightful as the data would suggest that if these metals drop out of the solution and get incorporated into the actual catalyst used in the HER, then the electrochemical activity of a given catalyst might be overestimated since contributions from these impurities are typically not accounted for. Since Fe has a high abundance in nature and is also typically used as a material in making electrochemical cells, we decided to see if Fe substitution or incorporation from the electrolyte into the main catalyst occurs and also to evaluate its impact on the HER activity of the catalyst. We followed a protocol in preparing a Fe-free electrolyte and the ICPMS results shown in Table 1 show that Fe was significantly reduced and typically Fe content less than 20ppm is considered Fe-free since Fe can’t be eliminated due to its high abundance in nature.
Table 1: Metal Profile for Unpurified and Fe-purified electrolytes.
| Electrolyte | 56Fe [#2] | 60Ni [#3] | 105Pd [#3] | 109Ag [#3] | 208Pb [#3] |
| Unpurified/Fe-containing (ppb) | 18,048 | BDL | 8,083 | 42,408 | 902 |
| Fe-free (ppb) | 6,454 | BDL | 22,023 | 12,858 | BDL |
Although the results also indicate that Pd is present in the sample, we think this might be highly unlikely for two reasons. First, on purifying the sample of Fe, the Pd concentration increases and secondly, our analytical sequence shows that high Pd-containing samples from our aminoboration study were analyzed before the KOH samples and since the blanks used in the washout still show the presence of Pd, then the Pd content observed may not originally be in our sample but rather from instrumental memory of previous samples analyzed. In addition, another observation from this report which was unplanned but very surprising is the significant decrease in the 105Ag and 208Pb concentration after Fe-purification which suggests that the Fe-purification protocol can be used or modified a little for Ag and Pb purification from KOH electrolyte.
Fe-substitution study
From the data reduction section in the results page, the best data were selected and plotted in Figure 1. The results from the ICP-MS analysis shown in Figure 1 for both the Fe-containing and Fe-free electrolytes indicate that as the catalyst carries out the reaction, the Fe content decreases with increasing potential cycling. This suggests that the Fe-impurity present within the sample decreases as Fe gets into the lattice structure of the Ni catalyst. However, for both types of electrolytes, before, and after each potential cycling, Ni wasn’t detected. The data suggests that the unpurified electrolyte doesn’t contain Ni impurity and while Fe impurity drops out of the solution, the Ni centers in the Ni-metals are not substituted and so the Fe is probably adsorbed on the surface of the Ni. Reasons for possible observation of adsorption rather than substitution could be evaluated from the method development approach. From the method design perspective, likely, the isotope used in evaluating Ni (60Ni) wasn’t the best since it was only ~26% abundant despite being free from interferences. Another possibility could be that the concentrations used for the calibration curve were too high to observe the trace dissolution of Ni that could occur due to Fe substitution.
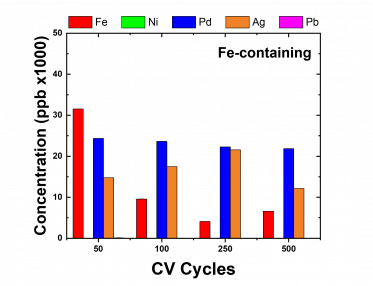
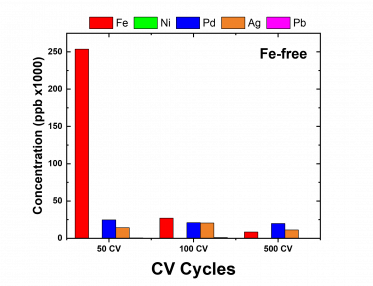
Figure 1: Metal profile of Fe-free and Fe-purified KOH electrolytes
Despite these, the results provide evidence that the presence of Fe impurity decreases with increasing potential cycling and could be responsible for activity decay in the HER. Hence one of the goals of the project which is to observe the occurrence of Fe-substitution was examined. Fe is probably adsorbed on the surface of the catalyst which possibly leads to a poor HER performance. Going forward, to improve the method, we can design a method that uses lower concentration calibration standards and also includes multiple blanks or avoid analyzing samples with long memory before the electrolytes.
Aminoboration
Table 2: Percentage of [Pd2+] measured as 105Pd present within supernatant for each additive as well as well as product formation determined by GC-FID.
| Additive | [Pd2+] (%) in supernatant | % product (GC-FID) |
| 0 mol % additive | 0.35 | Not Detected |
| 50 mol % Fe(OTf)3 | 2.47 | 33.06 |
| 50 mol % Cu(OTf)2 | 4.47 | Not Detected |
| 50 mol % Zn(OTf)2 | 2.10 | 6.22 |
| 10 mol % benzoquinone | 1.04 | 1.13 |
The effects of additives to leach solid-supported palladium is summarized in table 2 as relative % [Pd2+] in supernatant and precipitate. Comparing the percentage within supernatants for each, the sample with no additive had the lowest leached Pd content as expected. Interestingly, the leached Pd content using the Fe3+ source was similar to that of zinc, and well below that of the Pd content when using Fe(OTf)2 of 14.755%. The current mechanistic hypothesis is that an [Fe3+] species, generated in situ from Fe(OTf)2, is responsible for leaching within our system.13 While it is possible that the excess of Fe3+ in this case led to potential degradation of the iron species or otherwise engaged in varying side-reactions, it would be worthwhile to conduct further replicates for verification.
Furthermore, redox-inactive zinc showed a small percentage of leached Pd, which could suggest that other leaching mechanisms are contributing a minor concentration of Pd in solution. Benzoquinone in a small (10 mol%) amount relative to the other additives (50 mol%) provided Pd content in the supernatant above that of the sample with no additive, suggesting that it may have some ability as an oxidant. Notably, Cu(OTf)2 was the most efficient as compared to the other additives used in this study. Copper co-catalysts have been utilized as sources with which to re-oxidize Pd. Cu(OTf)2 similarly offered an excellent product yield for the norbornene substrate in reaction optimization (in situ yield the same as obtained for Fe(OTf)2), though the % leached Pd is still significantly lower than when using Fe(OTf)2.
Despite the discrepancy in leaching efficiency when using Fe(OTf)3, the ability of this additive to produce selective aminoboration product greatly exceeds that of the other species. Contrasting the leaching efficiency, copper serves as a poor co-catalyst for product formation when using solid-supported Pd, generating no product as compared to >95% in the optimized reaction conditions. Zinc is capable of forming small amounts of product, while BQ only provides trace amounts. Importantly, as expected, the background reaction with no additive produces no detectable product, following that of the optimized reaction being reliant on both the Pd catalyst source and the co-catalyst (additive) for aminoboration product.
Generally the leaching efficiency was as expected for the various screened additives. The enhanced efficiency with the Cu source, while potentially reasonable, should be investigated in replicate again and compared to replicates for Fe(OTf)3 as well to ensure accuracy. Additionally, future investigations should make comparisons for this series of additives using the alternative optimized substrate homoallylbenzene. Previous ICP-MS Pd leaching studies using this substrate indicated enhanced leaching with the same loading of Fe(OTf)2, though with lowered loading of Pd relative to norbornene. The reduced ability of large NPs to agglomerate may allow for greater access by these additives for re-oxidation to leach Pd back into solution. As such, it would be worthwhile to make the comparison between norbornene and homoallylbenzene across the additive series.