Evaluating the Metallic Profile of Electrolytes and Reaction Mixture in Heterogeneous Catalysis
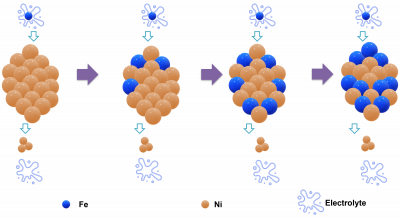
Electrolytes such as KOH as obtained from the vendors typically have metal impurities and sometimes when used in electrocatalysis, can lead to an overestimation of a catalyst’s performance. This is because the metal impurities in the electrolytes could also be catalyzing the reaction of interest and hence cause an analytical error in catalytic activity assessment. To ensure that catalytic performance reports are as accurate as possible, it’s important to have a bird-view of the metallic profile of the electrolytes to monitor metals that can probably improve the rate of reaction thereby catalyzing the reaction of interest.
Another goal is to establish a working comparison of various Lewis acids and oxidant species to bring palladium into solution from palladium nanoparticles (NPs). This expands on work found by Hull et al. in which a palladium and iron co-catalyzed system was established for the aminoboration of terminal and internal unactivated alkenes. In this system, FeIII generated over the course of the reaction from Fe(OTf)2 served to “leach” palladium back into solution, thereby re-activating the active catalyst. While Fe(OTf)2 provided the best oxidant for this purpose and product formation, establishing a comparison of various Lewis acidic additives and an organic oxidant.

Research Objectives
- Assessing the metallic composition of Ni, Fe, Ag, and Pb in potassium hydroxide electrolyte used in electrocatalysis before the reaction.
- Periodic appraisal of the foresaid metal content of potassium hydroxide electrolyte after each reaction cycle of interest.
- Measuring the metallic elemental composition of a heterogeneous catalyst
- Study efficiency of leaching Pd used in reaction into solution by varying additives for aminoboration reaction.