Background
Catalysts are materials that improve the rate of a chemical reaction by lowering the energy requirement needed to drive the reaction of interest. Some of these catalysts contain transition metals due to their d-orbitals that serve as a mediator, allowing for electron transactions necessary for reactions to occur. As a result, quantifying the amount of these transition metals in reaction systems is of chemical and economic value. To evaluate the role of transition metals as catalysts, qualitative and quantitative evidence is essential and quite a myriad of instrumental techniques are available for which the inductively coupled quadrupole mass spectrometry (ICP-Q-MS) stands out. A typical quadrupole ICP-Q-MS is shown in Figure 1 and possesses the fine attributes of rapid analysis, wide linear dynamic range, and allows for isotopic quantification in elemental analysis. In our study, we intend to develop a robust solution mode ICP-Q-MS method that can efficiently quantify Ni, Fe, Ag, Pb, and Pd in our reactions. To do this, a couple of useful ideas will be integrated into developing a method that will be useful for the aforementioned trace metal determination in our sample.
(a) (b)
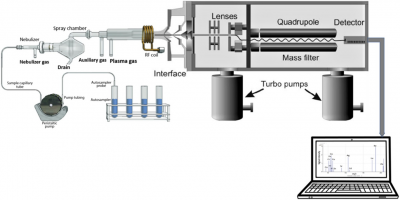

Figure 1: (a) An X-ray of a Typical Quadrupole ICP-MS 20 (b) External view of Agilent 7500 ce Quadrupole ICP-MS
Landing and co-workers had investigated the combined use of collision reaction cell (CRC), hot and cold plasma techniques for the detection and quantification of trace metals in a seawater matrix using an Agilent 7500cs. In their study, 56Fe, 75As, 78Se, and 111Cd were examined using both collision mode and reaction modes of a collision reaction cell. The outcome of their study indicates that the collision mode which utilizes helium as a kinetic energy discriminator in eliminating polyatomic interferences was unable to remove polyatomic interferences. However, the use of the reaction mode that made use of hydrogen as a reactant in breaking up plasma-based interferences proficiently eradicated these interferences. The reaction mode improved the sensitivity to matrix blank ratios for 56Fe, 78Se, and 75As which could be obscured by 40Ar16O+, 40Ar2, and 40Ar35Cl respectively. For specific metals such as 56Fe, cold plasma improved the sensitivity due to the decreased formation of ArO+.21 This highlights the need for CRC in multi-elemental analysis.
In addition, matrix type also influences the choice of methods used in evaluating the elemental component of a sample. This is because analytes of interest are locked within samples and need to be completely dissolved in solution to ensure sampling is statistically representative of the whole. For instance, to quantify the Fe, S, and Mn content in food samples, Renata et al first freeze-dried, and ground the animal and plant samples respectively before digesting the sample with nitric acid.22 A similar operation was observed in the study by Marcel and co-workers in which they dissolved 50mg of a meteorite sample before quantification of 26 elements.23 However, if a sample is already available in solution forms such as seawater, aqueous or organic mixture, it can be pretreated and aspirated directly into the instrument or pre-concentrated depending on the analytical need.24–26
While many studies apply the use of multiple metals such as Sc, Y, In, and Tl as internal standards in elemental analysis 27,28, it is the opinion of the authors that the use of single internal standards be encouraged. While this can be challenging, the use of single internals standards offers remarkable advantages of effectively minimizing contamination, and analytical cost while checking analyte signal or instrumental drift in a stellar fashion.29,30 Donati et al 31 proposed the use of a technique called the interference standard approach (IFS) which uses changes in Ar species from the plasma to check fluctuations in interference signals instead of the analyte signal as customary. The rationale of this technique stems from the fact that the Ar species from the plasma shares similar behavior with the interfering ions. Consequently, by normalizing the analytical signal (which is a sum of the analyte and interference signal) with the interference signal, the interfering signal can be minimized. Other studies have since utilized the IFS approach with the use of Xe 32, and Ar 22 which are plasma gases, and the results were remarkable falling within 4% RSD which indicates the usefulness of IFS.32
Interestingly, the matrix of the kind in this study (KOH) has been assessed for the presence of 60Ni, and 208Pb previously. As shown in Figure 2, in that work by Lu et al, an electro dialyzer was added to the sample introduction system which feeds into the nebulizer.33 The purpose which is to separate the K+ from the solution while neutralizing the OH– offers an online version of an ion-exchange resin rather than the offline to minimize contamination issues. Other studies have suggested the use of dilution of matrix to minimize the impact of alkaline matrixes such that by diluting with acids they can be neutralized and further dilution will keep the salts soluble enough to prevent the clogging of the nebulizer.
Our goal is to combine these techniques in such a way as to develop a robust solution mode ICP-Q-MS method with enviable analytical figures of merits in the quantification of Ni, Fe, Ag, Pb, and Pd. We intend to use CRC to minimize plasma-informed interferences, minimize the use of multiple internal standards, and create a well-diluted matrix that is clogging-free. Even more, accuracy and precision are essential watchwords for the analyst. To achieve this, quality control samples with concentrations different from the calibration curve will be added at intervals within the analytical scheme to periodically check for accuracy while precision will be monitored by repetitive measurements.
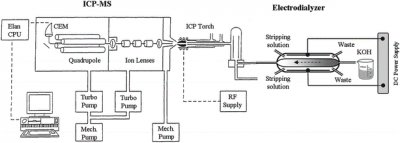
Figure 2: Online Ion Exchange Resin (Electro dialyzer) in the Sample Introduction System 33
ICP-MS analysis of noble metals is often employed for trace contaminant purposes, in which ppb concentrations are appropriate.19,26,35 Among these contaminant investigations, environmental sample applications are common, such as water contamination and road pollutants through these metal species, with potential negative impacts to soil quality and wildlife ecosystems.19,26,35 Another common application is in pharmaceutical-based studies to investigate metal distribution in biological systems and pathways.34
Typical ICP-MS analysis of Pd utilizes the combination of concentrated HCl and HNO3. These two acids, when combined in a 3:1 ratio of HCl to HNO3 create a strong oxidizing agent with the ability to dissolve most noble metals. This in combination with microwave digestion, allows for the complete digestion of solution-matrix samples. Typical Pd standards range from 0 ppb (blank) to 100 ppb for calibration ranges, and internal standards of Sc, Y, In, Tb, Bi have been used34, along with suggestions for the use of Re.19
The solution mode analysis of noble metals such as Rh, Pd, and Pt implicates 105Pd as being relatively free of isobaric Cd interference. (road dust ex) Varying polyatomic, isobaric, and doubly charged interferences are possible for Pd isotopes including 105Pd (see methodology for interference table) lending to the importance of using collision reactor cell (CRC) and He modes for quantification and comparison. Monitoring of significant interferences is treated as typical via T-ratios (threshold ratios)19, indicating how significant the spectral interference intensity occurs above the threshold signal of the blank. Laser Ablation ICP-MS (LA-ICP-MS) in comparison to High-Resolution ICP-MS (HR-ICP-MS) have also been used to analyze Pd concentrations, but interferences within the environmental samples used led to uncertainty for quantitative analysis. This study indicated that 105Pd was more accurate (less prone to significant interferences) compared to 106Pd, but that an 40Ar65Cu potentially contributed to the measurement of the former Pd isotope species.35