Proposal
Research Objectives & Hypothesis
The Snowball Earth Hypothesis comes from evidence of two severe glaciation periods on Earth during the Neoproterozoic era. These two Cryogenian glacial episodes are known as the Sturtian (~717-659 Ma) and Marinoan (~650-635 Ma) glaciations correspond to geologic evidence for a possible Snowball Earth with glacial ice sheets extending to the equator (Christie-Blick et al., 1999) (Hoffman and Li, 2009) (Prave et al., 2016) (Stern and Miller, 2019). If ice sheets entirely or substantially covered Earth, the atmosphere may have been primarily isolated from interaction exchange with the oceans, causing restrictions in the hydrologic cycle. The non-interaction between the atmosphere and oceans may culminate with factors that could change seawater’s chemical composition (Hoffman et al., 1998) (Christie-Blick et al., 1999). The geologic indicators for these glaciation events come from evidence of post-glaciation features such as glacial deposits (dropstones and diamictites) and deposition of cap carbonates across the globe (Fanning and Link, 2004) (Hoffman and Li, 2009) (Hoffman, P.F., 2011). Cap carbonates represent rapid changes towards greenhouse conditions presenting changing environmental conditions when the atmosphere-ocean exchange resumed after millions of years-long intervals. These changes in environmental conditions are reflected in the seawater composition. Atmospheric CO2 gradually increased from volcanic outgassing during these glacial time periods, causing melting of low latitude ice sheets, which resulted in global albedo decrease assisting with continuous melting of glacial ice sheets (Hoffman et al., 1998) (Christie-Blick et al., 1999) (Stern and Miller, 2019).
This study’s objective is to examine whether cap carbonates from different locations across the globe during specific glacial episodes (Sturtian & Marinoan) were deposited under similar or different environmental conditions. We propose to develop two methods (solution ICP-MS and LA-ICP-MS) to obtain elemental geochemistry and create chemostratigraphic correlations across different localities. We will analyze major and trace element concentrations to assess mineralogic composition (limestone vs. dolostone; Ca & Mg), sample preservation representing primary deposition (Al, K, Si), and redox proxies in seawater (Y and REEs). However, we predict all cap carbonate samples for a given glacial interval (Sturtian or Marinoan) to exhibit similar compositions, supporting the existence of Snowball Earth twice in the Neoproterozoic. In our hypothesis, we anticipate oceanic conditions being homogenized to globally anoxic conditions during the Tonian, Sturtian, and Marinoan glaciation events.
Elements to consider:
Major: 24Mg+, 27Al+,28Si+, 40Ca+,43Ca+, 44Ca+,39K+,55Mn+, 56Fe+,57Fe+
Trace: 7 Li+, 88Sr+, 97Mo+, 51V+
REEs: 89Y+, 139La+, 140Ce+, 141Pr+, 146Nd+, 147Sm+, 153Eu+, 157Gd+, 159Tb+, 163Dy+, 165Ho+, 166Er+, 169Tm+, 172Yb+, 175Lu+, 232 Th+, 238U+
Motivation
Recent studies show the significance of ICP-MS elemental characterization to better understand deglacial conditions. Johnson et al. (2017) conducted solution and LA-ICP-MS analysis to investigate redox sensitive trace element concentrations as a proxy for redox conditions during the Marinoan glaciation period. However, current studies often focus on one locality, and we seek to evaluate if redox proxies correlate among widely separated deglacial marine environments for cap carbonate successions following the Sturtian and Marinoan glaciations.
To investigate the redox state variations of the water column during Neoproterozoic deglaciation events, we will analyze the rare earth elements group. While terrestrial carbonates show low REEs concentrations, higher abundances observed on seafloor sediments indicate that REEs may serve as a proxies for seawater conditions during the depositional period (Banner et al. 1988, Tostevin et al. 2016). The REEs present very similar chemical properties and, consequently, they show shale-normalized smooth elemental distribution patterns (Pourmand et al. 2011). Elemental anomalies in such patterns due to redox oceanic variations inform us on the seawater conditions during the deposition period. A negative Ce anomaly occurs in oxic oceanic environments, where Ce (III) oxidizes to Ce (IV), reacting with manganese oxides and depleting the water column from Ce (German and Elderfield 1990). Europium, on the other hand, reduces from Eu (III) to Eu (II) in anoxic conditions, leading to a positive Eu anomaly in the REEs pattern (Tostevin et al. 2016).
Our research takes advantage of both solution and laser ablation modes to investigate both bulk composition of our samples, as well as spatial elemental variations at the microscopic level. Solution mode will be used to determine the bulk composition of samples from different localities by microdrilling the most preserved regions of the sample rocks. To obtain spatial elemental information of our samples, laser ablation will be conducted as it provides equally accurate concentration measurements with a lateral resolution in the order of microns. LA-mode will be conducted by scanning thin sections of our samples in the direction normal to the bedding planes to evaluate changes in seawater redox conditions during the deposition period of our samples. Small geological features of interest, such as dropstones, will also be analyzed. To further compare local and bulk elemental compositions variations, laser scan lines will be conducted in the thin section at approximately the same location as the microdrilled solution-mode regions.
Summary of Relevant Work
After reviewing several articles related to cap carbonates and their associated chemistries, analytes that were most frequently utilized were then cross referenced with studies conducted as to the characterization of those isotopes in the context of analysis via ICP-MS. Common elements included Sr, Fe, Y, and many REEs (Ce, Eu, Gd, etc).
Sr isotope composition serves as a proxy for deposition rates within the carbonate (Kyser, 1998; Cockeron, 2007) with low 87Sr/ 86Sr ratios generally indicating a rapid formation of carbonate units (James, 2001). Elements under the banner of REE+Y tend to be insightful when investigating redox sensitive trace elements in cap carbonates and can be used to “monitor environmental changes recorded by marine carbonates” (Rodler, 2016). Y anomalies generally show distinct differences in pre and post glacial values in platform sections. Positive Eu anomalies reflect hydrothermal vent activity and Ce analytes can be used as proxies for determining the degree of oxygenation in seawater; when both Ce and Eu anomalies are studied in the context of carbonates, the analytes can be used to discern whether a stratified water column existed and gain insight as to how oxic or anoxic marine environments were (Rodler, 2016).
Analyzing these elements via ICP-MS does not come without interference, however. In a study conducted by Xie (1994) an analysis of 27 trace elements found in Basalt BIR-1 found significant amounts of isobaric interference among REEs and REE oxides. For example, 157Gd is interfered with by 141Pr16O. This was again explored in Lichte (1987) where it was also motioned that lighter REEs may form polyatomic species when they encounter hydroxides, 18O isotopes, or chlorine, thereby interfering with heavier REE signals.
Suggestions made for circumventing some of these problems included augmenting the sample introduction parameters such as operating the nebulizer as 1.86 mL/ min and setting the gas flow rate to 1.0 L/ min. Additional optimizations included deviating from the manufacturer’s manual by using a slightly positive potential on the lens as opposed to a suggested negative potential, using a high salt content standard to minimize background noise, grounding the S-2 lens and setting the B lens to +5.5V. Setting the B lens to the chosen voltage was a strategic tactic used by Lichte (1987) because it gave the best compromise between achieving relatively equal sensitivity across all REE while also not losing a significant amount of sensitivity. To overcome any matrix effects Xie (1994) employed the use of pure elemental standards in the external calibration wherein each unknown was run unspiked and then spiked and the complied the data to be used to establish a base line for each analyte.
Materials and Methods
Theory and Analytes
In order to constrain Neoproterozoic oceanic redox conditions following alleged snowball Earth conditions, we will measure certain major, trace, and rare-earth (REE) elements. Our primary focus will be to measure Ce anomalies in cap carbonates that are plausibly associated with the Sturtian (717-659 Ma) and Marinoan (645-635 Ma) glaciations. Unlike most of the other REEs which are trivalent, Ce also exists in a tetravalent oxidation state. Under progressively more oxidized conditions Ce(IV) increases in abundance at the expense of Ce(III), and the overall Ce enrichment in certain phases will vary due to the significantly different partitioning behavior of Ce’s two oxidation states in oceanic environments (Kuhn et al., 1998). In an oxidized ocean Ce(IV) readily is incorporated into Fe/Mn oxide precipitates (whereas Ce(III) is not) thus leaving seawater from which carbonates precipitate depleted in Ce (Bau et al., 1996). When carbonates precipitate from this oxygenated seawater, they will therefore have lower normalized Ce concentrations with respect to the REEs of similar ionic radii, i.e., a negative Ce anomaly occurs. While the occurrence of a negative Ce anomaly eliminates the possibility of anoxic oceanic conditions, the absence of a negative Ce anomaly does not necessarily suggest anoxic conditions because Mn oxides may not always precipitate in near-shore environments (Tostevin et al., 2016). A confirmation of anoxic (reducing) oceanic conditions requires collaborating evidence for the paucity of oxygen, such as the occurrence of sulfide minerals like pyrite. Therefore, investigation of carbonate Ce anomalies and the existence of Fe2+ minerals allows a first-order characterization of a depositional environment as being either oxic or anoxic. The Ce anomaly will be quantified following the geometric relation of Lawrence et al. ( 2006) as used by Tostevin et al. (2016) (Equation 1).
Equation 1
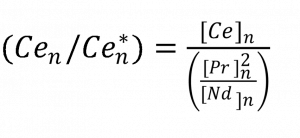
Evident from equation 1 is also the need to quantify the amount of Pr and Nd (REEs with similar ionic radii and partition coefficients to Ce(III) present in our Neoproterozoic carbonates. Additionally, other REE such as La, Eu , Gd, Lu and Y may offer further insight into carbonate depositional environment conditions (Allwood et al., 2010). Eu is particularly useful for our purposes because, like Ce, Eu exists in two valence states in oceanic waters: trivalent and divalent. Unlike Ce, Eu will have a pronounced positive anomaly in anoxic waters, as Eu2+ can readily substitute for Ca2+ in carbonate minerals. Likewise, measurement of Eu potentially offers an additional test for anoxic depositional conditions for our cap carbonates. Eu measurements via ICP-MS also necessitate the quantification of Ba, because BaO+ commonly interferes with both 151Eu+ and 153Eu+. Therefore, Ba concentrations will be analyzed as well to insure that our data is reflective of true Eu concentrations, and not just interference from Ba-rich samples. Additional trace elements such as Mn, Mo, V, and U can also vary in concentration in carbonates based off of oceanic redox state (Schrӧder and Grotzinger, 2007) and will likewise be measured here to supplement the Ce and Eu data. Whether or not our carbonate samples preserve their original depositional chemistry is also crucial to evaluate. Major elements such as Mg and Ca reveal how dolomitized our cap carbonates are. Al, Si, and Na will be measured to assess whether any aluminosilicate material may have been incorporated during the laser ablation or sample dissolution process. Trace element preservation will be assessed by measuring Y/Ho and calculating the bell shaped index (BSI, see equation 2), which is a measure of middle-REE enrichment in a carbonate sample (Tostevin et al., 2016).
Equation 2
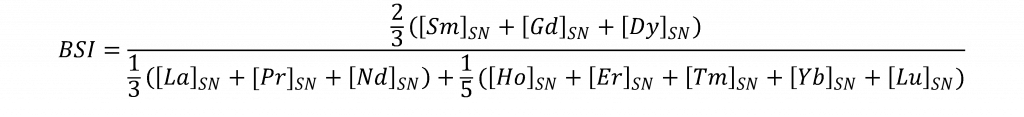
Where “SN” refers to shale normalized concentrations. The criteria for well-preserved samples is a high Y/Ho ratio and low BSI value, as unaltered carbonates have positive Y anomalies unlike silicates and oxides), but are depleted in MREE relative to diagenesis related phosphate or oxide precipitates (Tostevin et al., 2016; Wang et al., 2014; Huang et al., 2009).
The table below lists all of our target analytes and their interpretation purposes. Collectively, these elements will be used to confirm that carbonate chemistry is reflective of environmental conditions at the time of deposition, as well as, evaluate changes in oceanic redox conditions.
31 elements total
Table of Target Analytes
| Element | Target Isotope | Examples of Possible Interferences (Keating, 2004; Aries et al., 2000) |
Purpose |
| Li | 7Li+ | Trace element Composition | |
| Ca | 40Ca+**, 43Ca+** ,44Ca+* | 28SiO+, 27AlOH+, 40Ar+, 38ArH2+,24MgO+, 25MgO+ | Major element Composition |
| Mg | 24Mg+ | 6LiO+, | Major element Composition |
| K | 39K+ | 23NaO+ | Aluminosilicate Monitor |
| Si | 28Si+*, 29Si+** | 12CO+,14N2+, 12COH+,12CO+ | Aluminosilicate Monitor |
| Al | 27Al+ | Aluminosilicate Monitor | |
| Fe | 56Fe+*, 57Fe+** | 41KO+, 40KOH+, 40ArOH+ | Redox Sensitive |
| Mn | 55Mn | 39KO+, 38ArOH+ | Redox Sensitive |
| La | 139La+ | 123SbO+,123TeO+, 122SnOH+, 122TeOH+ | Depositional Environment Characterization, Preservation Indicator |
| Ce | 140Ce+, 140Ce++ | 124SnO+,124TeO+, 123SbOH+, 123TeOH+ | Redox Proxy, Oxide Production Monitor, Second Ionization Monitor |
| Pr | 141Pr+ | 125TeO+,124TeOH+, 124SnOH+ | Redox proxy, Preservation Indicator |
| Nd | 146Nd+ | 130BaO+, 130TeO+ | Redox Proxy, Preservation Indicator |
| Sm | 147Sm+ | 130BaOH+ | Redox Proxy, Preservation Indicator |
| Eu | 153Eu+ | 137BaO+,136BaOH+, 136CeOH+ | Redox Proxy |
| Gd | 157Gd+ | 141PrO+, 140CeOH+ | Depositional Environment Characterization, Redox proxy, Preservation Indicator |
| Tb | 159Tb+ | 143NdO+,142CeOH+, 142NdOH+ | Preservation Indicator |
| Dy | 163Dy+ | 147SmO+, 146NdOH+ | Preservation Indicator |
| Ho | 165Ho+ | 149SmO+, 146NdOH+ | Preservation Indicator |
| Er | 166Er+ | 150NdO+,150SmO+, 149SmOH+ | Preservation Indicator |
| Tm | 169Tm+ | 153EuO+,152SmOH+, 152GdOH+ | Preservation Indicator |
| Yb | 172Yb+ | 156GdO+,156DyO+, 155GdOH+ | Preservation Indicator |
| Lu | 175Lu+ | 159TbO+,158GdOH+, 158DyOH+ | Preservation Indicator |
| Y | 89Y+ | 62NiO+, 61NiOH+ | Depositional Environment Characterization |
| Ba | 135Ba+, 137Ba+, 138Ba+ | Interference Monitor | |
| V | 51V+ | 36ArN+,36ArNH+, 15NO2+, 14NO2H+ | Redox Sensitive |
| U | 238U+ | 222RnO+ | Redox Sensitive |
| Mo | 97Mo+ | 81BrO+, 80SeOH+ | Redox Sensitive |
| Sr | 88Sr+ | 72GeO+, 71GaOH+ | Depositional Environment Characterization |
*Solution Mode only, ** Laser Ablation Mode Only
Sample Suite
The sample suite under study comprises Neoproterozoic cap carbonate units from around the world (see map below).
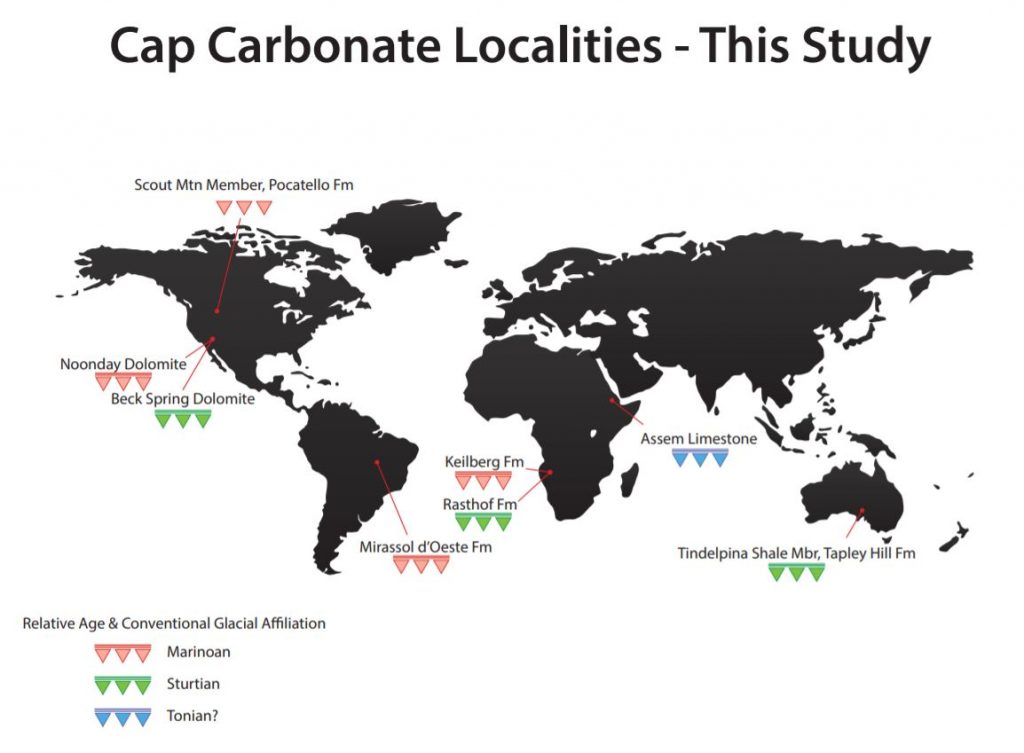
This sample suite consists of polished thin sections and associated billets. Laser ablation analysis will be used on the thin sections for targeted sampling, whereas the billets will be sampled via micro-drilling primarily to obtain representative bulk-carbonate compositions via solution mode analysis. Units following the Sturtian glaciation include: (1) the Rastof Formation (Namibia) and (2) the Tindelpina Shale Member of the Tapley Hill Formation (Australia). Units following the Marinoan glaciation include: (1) the Noonday Dolomite (Death Valley, USA), (2) the Keilberg Formation (Namibia), (3) the Scout Mountain Member of the Pocatello Formation (Idaho, USA), and (4) the Mirassol d’Oeste Formation (Brazil). Two pre-Sturtian carbonate units: the Beck Spring Dolomite (Death Valley, USA) and the Assem Limestone (Ethiopia), are also included in the sample suite. These two units may or may not be associated with the end of a glacial event. Nevertheless, they will be compared here with the Sturtian and Marinoan cap carbonates to assess how their origin may be similar or dissimilar to “true” cap carbonates.
We will analyze our target analytes in these cap carbonates using an inductively coupled plasma-quadruple-mass spectrometer (ICP-Q-MS). Both solution mode and laser ablation mode (LA) will be used for different purposes. Solution mode will be utilized primarily to determine time integrated bulk carbonate representative compositions. Laser ablation will be used to obtain time transgressive compositions of individual laminae, while avoiding obvious areas of a post-depositional nature such as fractures, veins, and areas that are visually chemically altered. Laser ablation will also be used on the Mirassol d’Oeste Formation samples to obtain representative bulk carbonate compositions because only thin sections are available to analyze for that unit.
Solution Mode Method
The primary purpose of the solution mode method is to obtain representative bulk carbonate compositions of our samples from each locality. Carbonate powders representative of lithologic variations at each locality were obtained from corresponding areas of billet samples via micro-drilling. A lengthwise trench perpendicular to bedding was drilled on most billets to obtain bulk compositions; however, for certain samples specific zones known to be unaltered carbonate of primary origin were drilled. Photo scans of billets both before and after micro-drilling are available on the “samples” tab for reference. At least 20 mg of carbonate powder were obtained from each area targeted for micro-drilling. This powder will be dissolved in two aliquots using acids of different strength following the method proposed by Miller (2012), which will be described briefly below.
Approximately 10 mg of powder will be distributed into two aliquots. The first aliquot will be dissolved in ~ 2.0 ml of HNO3 under agitation for 24 hours. This “strong acid” digestion is designed to dissolve all carbonate material. After dissolution the supernatant will be extract and placed into a clean vial for further dilution prior to ICP-MS analysis. The insoluble residue will tripled washed in nanopure H2O, dried, and weighed, such that the dissolved sample mass can be determined via equation 3.
Equation 3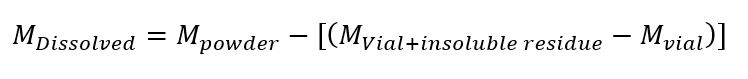
Where Mx is the mass of x. The initial dilution factor of the dissolved sample is calculated using equation 4.
Equation 4
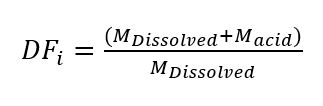
This supernatant will be further split into two additional aliquots. The first aliquot will be further diluted such that Ca is 200 ppm or less and will be used for trace element analysis. The second aliquot will be further diluted such that Ca is 10 ppm or less and will be used for major element analysis.
This same process will be followed on the second 10 mg aliquot of powder as well. This powder aliquot will be digested using ~ 2.0 mL of 0.345 M CH3COOh under agitation for ~24 hours. This “weak acid” digestion is designed to only dissolve ~80% of the carbonate fraction of the powder; therefore, insuring that aluminosilicate contamination is eliminated or minimized. The remaining process is exactly the same as for the strong acid digestion, with two aliquots of dissolved powder with distinct dilution factors for trace and major element analyses. The purpose of diluting with both weak and strong acid is to make sure that what we are sampling is entirely carbonate material. The strong acid will dissolve all carbonate and will be more representative of the carbonate chemistry given that no aluminosilicates are dissolved. In that case of aluminosilicate contamination, the weak acid digestion will be more representative of the carbonate chemistry that we aim to determine.
After digestion, the dissolved samples will be analyzed using solution mode ICP-Q-MS for the target analytes as described above. Production of polyatomic ions will be monitored by comparison 140CeO+/Ce+, with an optimal ratio of 1% or less. Production of doubly charged species will be monitored using Ce (m/z= 140 for singly charged; m/z=70 for doubly charged.Polyatomic interferences will be reduced using a collision reaction cell running in either H2 (reaction) or He (collision) gas mode depending on the target ion. Following calibration with an external standard solution, a sequence of method blanks, quality control standards, and spiked unknowns, will be interspersed within the unknown solutions. Each unknown will be spiked with a Be internal standard prior to sample introduction. Continued monitoring of standards, and internal standards, throughout the run will ensure that precision remains adequate through the analytical sequence, as well as track any machine drift that may occur. The method blanks serve as a monitor for inherent laboratory contamination introduced during the sample preparation and/or analytical process. Finally, the analyses of spiked unknowns will test the accuracy of our results.
The ICP operating conditions for the solution mode method will be based off of the recommendations for optimizing signal and sensitivity as outlined in Table 1 of Lichte et al. (1987). These parameters will be adjusted to minimize oxide and doubly charged species production some for our ICP instrument. Lichte et al. (1987) recommends RF power to be set at 1600 W with an associated sheath Ar-gas flow of 0.25 L/min to minimize oxide production. Our sample introduction rate will follow the 1.86 mL/min of Lichte et al. (1987); however, our nebulizer gas flow rate will be set at 1.29 L/min (instead of 1.0 L/min) to optimize for the use of nitric acid as our sample matrix (Longerich, 1989). The remaining parameters will be in accordance with Lichte et al. (1987) with a plasma gas flow rate of 13 L/min, a sampling distance of 15 mm, and a measurement dwell time of ~200 ms.
Laser Ablation Mode Method
The primary purpose of the laser ablation analytical method is to analyze features that are too small to be microdrilled without introducing contamination from adjacent post-depositional or non-carbonate phases. Laser ablation enables time transgressive sequences of laminations and interesting microscopic features, such as a dropstones, to be analyzed in-sotu on thin sections. Laser ablation will also be used to determine bulk representative compositions for Mirassol d’Oeste Formation due to a lack of billets available for micro drilling.
The specific isotopes measured for each of our target analytes for our laser-ablation analysis are slightly different than for solution mode (see above table). The collision reaction cell may only be run in no gas mode during laser ablation analyses, so there is less flexibility in targeting certain ions with common interferences. Oxide and divalent ion production will be minimized during tuning, with oxide interferences monitored using ThO/Th on the NIST 612 calibration standard. For standardization, we will use USGS MACS-3 as our calibration standard, NIST 612 as our external reference standard, and Ca as an internal reference. We will ablate our samples using an ESL NWR 193 nm excimer laser in a helium atmosphere with a gas flow rate of ~0.8L/min. Tanaka et al. (2007) demonstrates that using a He, rather than Ar, atmosphere for the ablation process results in more accurate measurements in REE concentrations due to the production of smaller aerosols with less size variance. Laser ablation spot sizes will vary based on the size of the feature to be analyzed, but will range from 20-100 µm in diameter The pulse width of the laser will be 4-6 ns and the sampling depth will be 6 mm. The remaining ICP parameters will be similar to those used for solution mode: RF power=1600 watts, nebulizer gas flow rate of 1.29 L/min, auxiliary gas flow rate of 0.25 L/min, plasma gas flow rate of 13 L/min, sample introduction rate of 1.86 mL/min, sampling distance of 15 mm, and measurement dwell time of 200 ms (Longerich, 1989; Lichte et al., 1987).
Possible Outcomes
The product of this project will be a Neoproterozic cap carbonate geochemical dataset that describes that carbonate mineralogy, preservation of original carbonate chemistry, and the oceanic redox state as either oxic or anoxic during carbonate precipitation. The utility of this dataset depends upon how well our cap carbonate samples have preserved their original depositional conditions in their chemistry, which will be evaluated as part of our proposed work. Even in the presence of significant alteration, some critical chemical characteristics may still reflect of the initial depositional environment. For example, Liu et al. (2019) found that Ce anomalies in marine carbonates are not affected by common alteration processes such as dolomitization or post-depositional diagenetic processes involving water of a different composition than the seawater at the time of deposition. Once samples whose original composition has not been preserved have been filtered out, our dataset will be used to estimate oceanic redox conditions at the deposition using redox sensitive REEs (Ce, Eu) and possibly other trace elements (V, Mo, U). Collectively, this dataset will contribute to the existing knowledge of Neoproterozic cap carbonates and the depositional environments in which they formed.
An additional objective of our work is to globally compare the composition of cap carbonates associated with the same preceding Neoproterozoic glaciation. This comparison will test whether the environments in which each cap carbonate formed were similar or dissimilar. A dissimilar interpretation does not necessarily disprove the existence of snowball Earth during either the Sturtian or Marinoan glacial episodes, as even if the ocean was sealed off from the atmosphere regional heterogeneities could exist in such a large reservoir. Dissimilar compositions might also result if Sturtian/Marinoan glaciations were regional in extent, as they are today, or if multiple glaciations occurred within the broadly defined Sturtian and Marinoan timeframes. Alternatively, if all cap carbonates sampled for a given glacial interval (Sturtian or Marinoan) have similar compositions, then this would support the existence of a snowball Earth at least twice in the Neoproterozoic. Under this scenario oceanic conditions were remarkably homogenized to globally anoxic conditions for extremely long time periods (107 years). Either outcome from our data interpretations would offer interesting insight to oceanic conditions on the Earth leading up to the Cambrian Explosion.
A final benefit from our proposed work will be the establishment of two analytical methods for analysis of carbonate rocks using ICP-Q-MS. These methods will analyze the same suite of elements (REEs, other trace elements, and major elements) but differ in sample delivery; with one being designed for solution mode and the other for laser ablation mode. Other researchers may find these methods useful for analyzing carbonate samples in general, regardless of age or preservation.
Timeline and Budget
| Sample Type | Cost (USD) |
| Solution Mode | 527.00 |
| LA Spot | 60.83 |
| LA Line | 36.50 |
| Method Development | 146.00 |
| Instrument optimization | 109.50 |
| Total Cost | 879.83 |
| Sept ’20 | Oct ’20 | Nov ’20 | Dec ’20 | ||||||||||||
| Literature Review | X | X | X | X | |||||||||||
| Refine Methodology | X | X | X | ||||||||||||
| Sample Spot Location | X | X | X | ||||||||||||
| Sample Preparation | X | X | X | ||||||||||||
| Data Collection | X | X | |||||||||||||
| Data Analysis | X | X | |||||||||||||
| Write and Submit Report | X | X | X | ||||||||||||
References
Alene, Mulugeta, et al., 2006, The Tambien Group, Ethiopia: An Early Cryogenian (Ca. 800–735Ma) Neoproterozoic Sequence in the Arabian–Nubian Shield: Precambrian Research, vol. 147, no. 1-2, pp. 79–99., doi:10.1016/j.precamres.2006.02.002.
Allwood, A.C., Kamber, B.S., Walter, M.R., Burch, I.W., and Kanik, I., 2010, Trace elements record depositional history of an Early Archean stromatolitic carbonate platform: Chemical Geology, v. 270, no. 1-4, p. 148–163, doi:10.1016/j.chemgeo.2009.11.013.
Aries, S., Valladon, M., Polvé, M., and Dupré, B., 2000, A routine method for oxide and hydroxide interference corrections in ICP-MS chemical analysis of environmental and geological samples: Geostandards Newsletter, v. 24, no. 1, p. 19–31, doi:10.1111/j.1751-908X.2000.tb00583.x.
Bau, M., Koschinsky, A., Dulski, P., and Hein, J.R., 1996, Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater: Geochimica et Cosmochimica Acta, v. 60, no. 10, p. 1709–1725, doi:https://doi.org/10.1016/0016-7037(96)00063-4.
Beyth, M., 2001, Preliminary indications for Snowball Earth in the East African Orogen: Geological Society of Australia Abstracts, v. 65.
Bosak, T., Lahr, D.J., Pruss, S.B., Macdonald, F.A., Gooday, A.J., Dalton, L. and Matys, E.D., 2012. Possible early foraminiferans in post-Sturtian (716− 635 Ma) cap carbonates. Geology, 40(1), pp.67-70.
Christie-Blick, N., Sohl, L. E., & Kennedy, M. J. (1999). Considering a neoproterozoic snowball Earth. Science. https://doi.org/10.1126/science.284.5417.1087a
Corkeron, M., 2007, ‘Cap carbonates’ and Neoproterozoic glacigenic successions from the Kimberley region, north-west Australia: Sedimentology, v. 54, p. 871–903, doi: 10.1111/j.1365-3091.2007.00864.x
Corsetti, F.A., and Grotzinger, J.P., 2005, Origin and significance of tube structures in Neoproterozoic post-glacial cap carbonates: Example from Noonday Dolomite, Death Valley, United States: Palaios, v. 20, p. 348–362, doi:10.2110/palo.2003.p03-96.
Corsetti, F.A., and Kaufman, A.J., 2003, Stratigraphic investigations of carbon isotope anomalies and Neoproterozoic ice ages in Death Valley, California: Bulletin of the Geological Society of America, v. 115, p. 916–932, doi:10.1130/B25066.1.
Corsetti, F.A., and Lorentz, N.J., 2006, On Neoproterozoic Cap Carbonates as Chronostratigraphic Markers, in Xiao, S. and Kaugman, A.J. eds., Neoproterozoic Geobiology and Paleobiology, Dordrecht, Springer, p. 273–294, doi:10.1007/1-4020-5202-2_9.
Cox, G., Isakson, V., Hoffman, P., Gernon, T., Schmitz, M., Shahin, S., Collins, A., Preiss, W., Blades, M., Mitchell, R., & Nordsvan, A. (2018). South Australian U-Pb zircon (CA-ID-TIMS) age supports globally synchronous Sturtian deglaciation. Precambrian Research, 315, 257–263. https://doi.org/10.1016/j.precamres.2018.07.007
Creveling, J.R., Bergmann, K.D., and Grotzinger, J.P., 2016, Cap carbonate platform facies model, Noonday Formation, SE California: Bulletin of the Geological Society of America, v. 128, no. 7/8, p. 1249–1269, doi:10.1130/B31442.1.
Crittenden et al., 1952, Parleys Canyon to Traverse Range; Geology of the Wasatch Mountains east of Salt Lake City, in Geology of the Central Wasatch Mountains, Utah: Utah Geol. Soc. Guidebook to Geology of Utah, no. 8, p. 1-37.
Crittenden et al., 1971, Nomenclature and correlation of some upper Precambrian and basal Cambrian sequences in western Utah and southeastern Idaho: Geol. Soc. America Bull., v. 82, no. 3, p. 581- 602.
Fanning, C.M., Link, P.K., 2003. Late Sturtian U–Pb SHRIMP age for Neoproterozoic Diamictites of the Pocatello Formation, southeastern Idaho: Geol. Soc. America Abstracts with Programs v. 35 no. 6, p. 389.
Font, E., Nédélec, A., Trindade, R., Macouin, M., & Charrière, A. (2006). Chemostratigraphy of the Neoproterozoic Mirassol d’Oeste cap dolostones (Mato Grosso, Brazil): An alternative model for Marinoan cap dolostone formation. Earth and Planetary Science Letters, 250(1), 89–103. https://doi.org/10.1016/j.epsl.2006.06.047
Frakes, L.A., and Crowell, J.C., 1967, Facies and Paleogeography of the Late Paleozoic diamictite, Falkland Islands: Geol. Soc. America Bull., v. 78, no. 1, p. 37-57.
Giddings, J., & Wallace, M. (2009). Sedimentology and C-isotope geochemistry of the “Sturtian” cap carbonate, South Australia. Sedimentary Geology, 216(1), 1–14. https://doi.org/10.1016/j.sedgeo.2009.01.007
Gyollai, I., Polgari, M., Fintor, K., Pal-Molnar, E., Popp, F. and Koeberl, C., 2017. Microbial activity records in Marinoan Snowball Earth postglacial transition layers connecting diamictite with cap carbonate (Otavi Group, NW-Namibia). Austrian Journal of Earth Sciences, 110(1).
Hoffman, P. F. (2011). Strange bedfellows: Glacial diamictite and cap carbonate from the Marinoan (635 Ma) glaciation in Namibia. Sedimentology, 58(1), 57–119. https://doi.org/10.1111/j.1365-3091.2010.01206.x
Hoffman, P.F., and Li, Z.-X., 2009, A palaeogeographic context for Neoproterozoic glaciation: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 277, p. 158–172, doi:10.1016/j.palaeo.2009.03.013.
Hoffman, P. F., Kaufman, A. J., Halverson, G. P., & Schrag, D. P. (1998). A Neoproterozoic snowball earth. science, 281(5381), 1342-1346.
Huang, J., Chu, X., Chnag, H., and Feng, L., 2009, Trace element and rare earth element of cap carbonate in Ediacaran Doushantuo Formation in Yangtze Gorges: Chinese Science Bulletin, v. 54, p. 3,295-3,302, doi: https://doi.org/10.1007/s11434-009-0305-1.
James, N.P., Narbonne, G.M., and Kyser, T.K., 2001, Late Neoproterozoic cap carbonates: Mackenzie Mountains, northwestern Canada: precipitation and global glacial meltdown: Canadian journal of earth sciences, v. 38, p. 1229–1262, doi: 10.1139/cjes-38-8-1229
Johnson, B.W., Poulton, S.W. and Goldblatt, C., 2017. Marine oxygen production and open water supported an active nitrogen cycle during the Marinoan Snowball Earth. Nature communications, 8(1), pp.1-10.
Kasemann, S. A., Hawkesworth, C. J., Prave, A. R., Fallick, A. E., & Pearson, P. N. (2005). Boron and calcium isotope composition in Neoproterozoic carbonate rocks from Namibia: Evidence for extreme environmental change. Earth and Planetary Science Letters, 231(1–2), 73–86. https://doi.org/10.1016/j.epsl.2004.12.006
Keating, G., 2004, Isotope Abundances and Interferences: Perkin Elmer, 14p.
Kuhn, T., Bau, M., Blum, N., and Halbach, P., 1998, Origin of negative Ce anomalies in mixed hydrothermal-hydrogenetic Fe-Mn crusts from the Central Indian Ridge: Earth and Planetary Science Letters, v. 163, no. 1-4, p. 207–220, doi:10.1016/S0012-821X(98)00188-5.
Kyser, T.K., James, N.P., and Bone, Y., 1998, Alteration of Cenozoic cool-water carbonates to low-Mg calcite in marine waters, Gambier Embayment, South Australia: Journal of sedimentary research, v. 68, p. 947–955, doi: 10.1306/D42688BA-2B26-11D7-8648000102C1865D
Lawrence, M.G., Greig, A., Collerson, K.D., and Kamber, B.S., 2006, Rare earth element and yttrium variability in South East Queensland waterways: Aquatic Geochemistry, v. 12, no. 1, p. 39–72, doi:10.1007/s10498-005-4471-8.
Le Heron, D., Cox, G., Trundley, A., & Collins, A. (2011). Two Cryogenian glacial successions compared: Aspects of the Sturt and Elatina sediment records of South Australia. Precambrian Research, 186(1-4), 147–168. https://doi.org/10.1016/j.precamres.2011.01.014
Lechte, M.A., Wallace, M.W. and Hoffmann, K.H., 2019. Glacio-marine iron formation deposition in a c. 700 Ma glaciated margin: insights from the Chuos Formation, Namibia. Geological Society, London, Special Publications, 475(1), pp.9-34.
Lichte, F.E., Meier, A.L., and Crock, J.G., 1987, Determination of the rare-earth elements in geological materials by inductively coupled plasma mass spectrometry: Analytical chemistry, v. 59, p. 1150–1157,
Link, P.K., 1983. Glacial and tectonically influenced sedimentation in the Upper Proterozoic Pocatello Formation, southeastern Idaho. In: Miller, D.M., Todd, V.R., Howard, K.A. (Eds.), Tectonic and Stratigraphic Studies in the Eastern Great Basin. Geological Society of America Memoir v. 157, p. 165–181.
Liu, X.-M., Hardisty, D.S., Lyons, T.W., and Swart, P.K., 2019, Evaluating the fidelity of the cerium paleoredox tracer during variable carbonate diagenesis on the Great Bahamas Bank: Geochimica et Cosmochimica Acta, v. 248, p. 25–42, doi:https://doi.org/10.1016/j.gca.2018.12.028.
Lorentz, J.L., 2003, Seafloor precipitates and C-isotope stratigraphy from the Neoproterozoic Scout Mountain Member of the Pocatello Formation, southeast Idaho: implications for Neoproterozoic earth system behavior, Precambrian Research, v. 130, p. 57-70.
Ludlam, J.C., 1942, Pre-Cambrian formations at Pocatello, Idaho: Jour. Geol., v. 50, no. 1, p. 85-95
Macdonald, F.A., Prave, A.R., Petterson, R., Smith, E.F., Pruss, S.B., Oates, K., Waechter, F., Trotzuk, D., and Fallick, A.E., 2013, The Laurentian record of Neoproterozoic glaciation, tectonism, and eukaryotic evolution in Death Valley, California: Geological Society of America Bulletin, v. 125, no.7-8, p. 1,203-1,223, doi: https://doi.org/10.1130/B30789.1.
Marian, M.L., and Osborne, R.H., 1992, Petrology, petrochemistry, and stromatolites of the Middle to Late Proterozoic Beck Spring Dolomite, eastern Mojave Desert, California: Canadian Journal of Earth Sciences, v. 29, no. 12, p. 2595–2609, doi:10.1139/e92-206.
Mbuyi, K., and Prave, A.R., 1993, Unconformities in the mid-Late Proterozoic Pahrump Group: Stratigraphic evidence from the upper member Crystal Spring Formation: Geological Society of America Abstracts with Programs, Reno, v. 25, no.5, p. 116.
Nogueira, A., Riccomini, C., Sial, A., Moura, C., & Fairchild, T. (2003). Soft-sediment deformation at the base of the Neoproterozoic Puga cap carbonate (southwestern Amazon craton, Brazil): Confirmation of rapid icehouse to greenhouse transition in snowball Earth. Geology (Boulder), 31(7), 613–. https://doi.org/10.1130/0091-7613(2003)031<0613:SDATBO>2.0.CO;2
Nogueira, A., Riccomini, C., Sial, A., Moura, C., Trindade, R., & Fairchild, T. (2007). Carbon and strontium isotope fluctuations and paleoceanographic changes in the late Neoproterozoic Araras carbonate platform, southern Amazon craton, Brazil. Chemical Geology, 237(1), 168–190. https://doi.org/10.1016/j.chemgeo.2006.06.016
Petterson, R., Prave, A.R., Wernicke, B.P., and Fallick, A.E., 2011, The neoproterozoic Noonday formation, Death Valley region, California: Bulletin of the Geological Society of America, v. 123, p. 1317–1336, doi:10.1130/B30281.1.
Prave, A.R., Condon, D.J., Hoffmann, K.H., Tapster, S. and Fallick, A.E., 2016. Duration and nature of the end-Cryogenian (Marinoan) glaciation. Geology, 44(8), pp.631-634.
Pruss, S. B., Bosak, T., Macdonald, F. A., McLane, M., & Hoffman, P. F. (2010). Microbial facies in a Sturtian cap carbonate, the Rasthof Formation, Otavi Group, northern Namibia. Precambrian Research, 181(1–4), 187–198. https://doi.org/10.1016/j.precamres.2010.06.006
Rodler, A.S., Frei, R., Gaucher, C., and Germs, G.J.B., 2016, Chromium isotope, REE and redox-sensitive trace element chemostratigraphy across the late Neoproterozoic Ghaub glaciation, Otavi Group, Namibia: Precambrian research, v. 286, p. 234–249, doi: https://doi.org/10.1016/j.precamres.2016.10.007
Schroder, S., and Grotzinger, J.P., 2007, Evidence for anoxia at the Ediacaran-Cambrian boundary: the record of redox-sensitive trace elements and rare earth elements in Oman: Journal of the Geological Society, v. 164, no. 1, p. 175-187, doi: https://doi.org/10.1144/0016-76492005-022.
Smith, L.H., Kaufman, A.J., Knoll, A.H., Link, P.K., 1994. Chemostratigraphy of predominantly siliciclastic Neoproterozoic successions: a case study of the Pocatello Formation and lower Brigham Group, Idaho, USA. Geol. Magazine v. 131, no. 3, p. 301–314.
Stern, R.J. and Miller, N.R., 2019. Neoproterozoic Glaciation—Snowball Earth Hypothesis. Age (Ma), 632(1.0), pp.632-3.
Swanson-Hysell, et al., 2015, Stratigraphy and geochronology of the Tambien Group, Ethiopia: Evidence for globally synchronous carbon isotope change in the Neoproterozoic: Geology, v. 43, p. 323-326, doi:10.1130/G36347.1.
Tanaka, K., Takahashi, Y., and Shimizu, H., 2007, Determination of rare earth element in carbonate using laser-ablation inductively-coupled plasma mass spectrometry: An examination of the influence of the matrix on laser-ablation inductively-coupled plasma mass spectrometry analysis: Analytica Chimica Acta, v. 583, no. 2 p. 303–309, doi:10.1016/j.aca.2006.10.023.
Tostevin, R., Shields, G.A., Tarbuck, G.M., He, T., Clarkson, M.O., and Wood, R.A., 2016, Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings: Chemical Geology, v. 438, p. 146–162, doi:10.1016/j.chemgeo.2016.06.027.
Trimble, D. E., 1976, Geology of the Michaud and Pocatello Quadrangles, Bannock and Power Counties, Idaho: Geol. Sur. Bull., 1400.
Wang, Q., Lin, Z., and Chen, D., 2014, Geochemical constraints on the origin of Doushantuo cap carbonates in the Yangtze Gorges area, South China: Sedimentary Geology, v. 304, p. 59-70, doi: https://doi.org/10.1016/j.sedgeo.2014.02.006.
Xie, Q., Jain, J., Sun, M., Kerrich, R., and Fan, J., 1994, Icp-ms analysis of basalt Bir-1 for trace elements: Geostandards and geoanalytical research, v. 18, p. 53–63, doi: 10.1111/j.1751-908X.1994.tb00504.x
