Sulfur Caves
Sulfidic Caves, Karst & Sulfuric Acid Speleogenesis

Microorganisms use reduced and oxidized forms of sulfur compounds to gain energy, while some heterotrophic microbes use sulfur compounds as electron acceptors during anaerobic respiration. Oxidation of reduced sulfur compounds, as hydrogen sulfide or other intermediate forms, can occur abiotically or biotically, whereby the final oxidation product is sulfate, commonly in the form of sulfuric acid. This acid has tremendous impact on the environmental surroundings of the microorganisms by causing extremely low pH conditions.
Global Distribution of Sulfidic Habitats
There are many different places around the world that are characterized by sulfidic waters, and therefore these places are habitats where microorganisms that utilize sulfur forms of energy will thrive. One of the most well-known and studied examples of a sulfidic environment is in hot springs, particularly those at Yellowstone National Park, Wyoming.
Active caves with H2S-rich groundwater has been reported in the literature include:
- United States: Cesspool Cave, Virginia; Crystal Beach Cave, Florida; Lower Kane Cave, Wyoming; Parker Cave, Kentucky; Deep portions of the Edwards Aquifer, Texas; Big Sulphur Cave, Kentucky
- Canada: “Sulfur” Cave, Banff National Park
- Mexico: Cueva de Villa Luz; Misc. cenotes
- Italy: Frasassi Caves (Grotta Grande del Vento-Grotta de Fiume); Submarine Caves
- Turkmenistan: Cupp-Coutum Cave
- Australia: Odesssy Cave
The Sulfuric Acid Speleogenesis Model
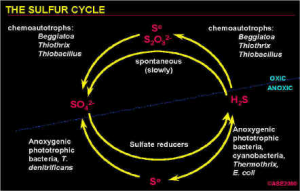
Waters can become enriched with hydrogen sulfide in the deep subsurface, possibly from biogenic activity such as dissimilatory sulfate reduction and petroleum reservoirs, or from volcanic sources. When this reduced, mineralized water rises to the surface, it cools and mixes with waters having different concentrations of hydrogen sulfide, and with meteoric waters that are often times oxidizing.
H2SO4 + CaCO3 + H2O –> CaSO4. 2H2O + CO2
The sulfide-rich waters will become oxidized and the most aggressive byproduct of sulfide oxidation, whether biologically-mediated or not, is sulfuric acid. Carbonate rocks will dissolve in acids, and the sulfuric acid increases carbonate solubility and porosity development along the zone of oxidation. Cavernous porosity can be created from this process, and the caves that have formed from this mechanisms have large chambers with narrow connections and deep shafts along avenues of sulfide water movement toward the surface. The most well-studied examples of cave systems having had a sulfuric acid speleogenesis are the caves of the Guadalupe Mountains in New Mexico and Texas.
For more information, Carlsbad Cavern National Park

