Methodology

The concentrations of Na, Mg, K, Al, Fe, Cr, and Zn were measured on Thursday, December 5th, 2024, using an Agilent 7500ce ICP-MS located at the Jackson School of Geological Sciences, University of Texas at Austin. The CeO/Ce ratio was determined to be below 1.05%, indicating optimal plasma temperature for maximum sensitivity and efficient breakdown of polyatomic interferences such as plasma-based oxides. To further mitigate polyatomic interferences, the octupole reaction system was operated in both helium and hydrogen modes. Additional quality control measures, including the use of internal standards to monitor instrumental drift, were implemented. The results showed that the internal standard sensitivity remained within the acceptable quality assurance tolerance range of 60-160%.
Table X provides the operating conditions and instrument parameters for the ICP-MS analysis.
Research Objectives
The goal of this study is to develop a method capable of accurately quantifying the concentrations of metallic elements (such as Mg and Fe) in olivine and the electrolytes involved in serpentinization reactions, particularly those affecting hydrogen production. The specific objectives are as follows:
- Evaluate the concentration of Mg, Fe, and other trace elements in different olivine samples before and after serpentinization reactions at varying pH levels, focusing on how these elements influence hydrogen production.
- Assess the impact of metallic impurities in olivine (e.g., Ni, Cr) on the serpentinization process and their potential role in hydrogen generation.
- Determine analytical figures of merit (such as LOD, LOQ, and LOL) for each analyte, ensuring accurate and precise quantification of trace elements.
- Optimize instrumental conditions for the effective determination of Mg, Fe, and other relevant trace metals in olivine during the serpentinization process.
- Evaluate the precision and accuracy of the method through repeated measurements, ensuring reliable data for further analysis of the metal’s role in catalyzing hydrogen production.
This approach will help elucidate the roles of key trace elements in serpentinization and contribute to understanding their impact on hydrogen production in natural and engineered systems.
Electrolyte Samples
I used DI water for two reactions and pH 11 was prepared for two other reactions. It was prepared using a solution of 50% NaOH and diluted with water using the equation: C1V1=C2V2 where: C=concentration and V=volume
The high pH solutions can’t go through the ICPMS directly, so:
- The samples were put in Savillex digestion vessels on a hot plate overnight at 70 °C in a fume hood.
- After they were completely dried, 1 mL of 65-70% conc. HNO3 was added to the leftover in the vessel and was taken for analysis.
- The samples were run in 1000x, 100x, and 25x dilutions.
Instrumental Conditions
Tuning:
Tuning was done to the instrument for no gas mode, then He mode, then H2 mode:
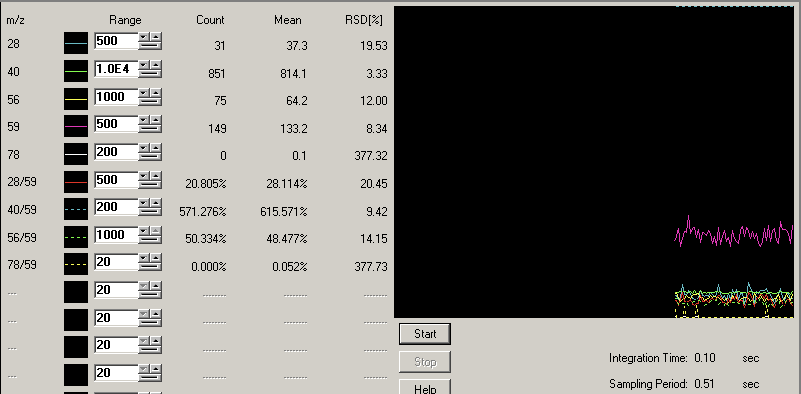
1- No gas tune:
torch location was adjusted.
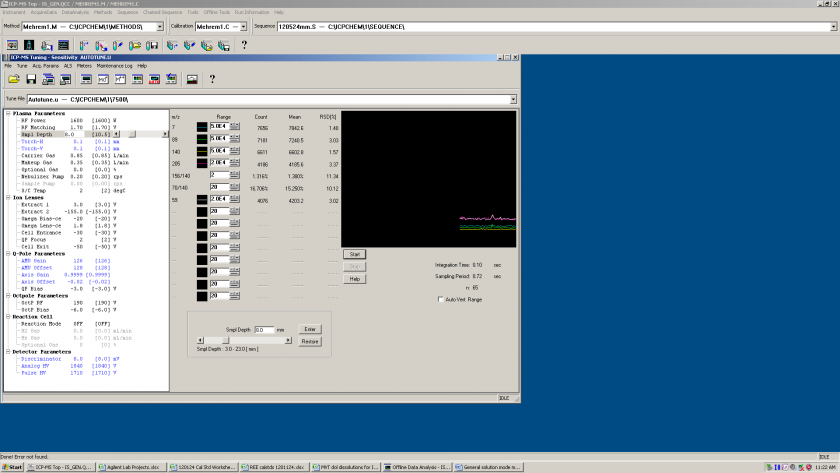
2- He rate adjustment:
We increased the rate to suppress the polyatomic interferences and we found it worked best at 5.5 mL/minute.
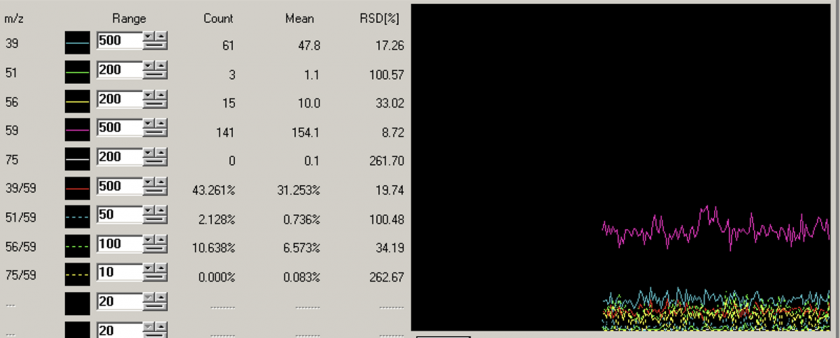
3- H2 Flow rate optimization:
H2 optimal flowrate was found to be 5 mL/minute.