Discussion/Major Findings
Serpentinization is a hydrothermal reaction in which olivine, a magnesium-iron silicate, reacts with water to form serpentine minerals, releasing hydrogen in the process. This reaction is of great interest due to its potential for hydrogen production, which is a key area of study for energy applications. The investigation of serpentinization at different pH levels provides insight into how the reaction dynamics and the concentration of metal ions such as magnesium (Mg) and iron (Fe) are influenced by environmental conditions. In this study, we analyze the concentrations of Mg and Fe using Inductively Coupled Plasma Mass Spectrometry (ICP-MS), focusing on two distinct pH conditions: 7 and 11.
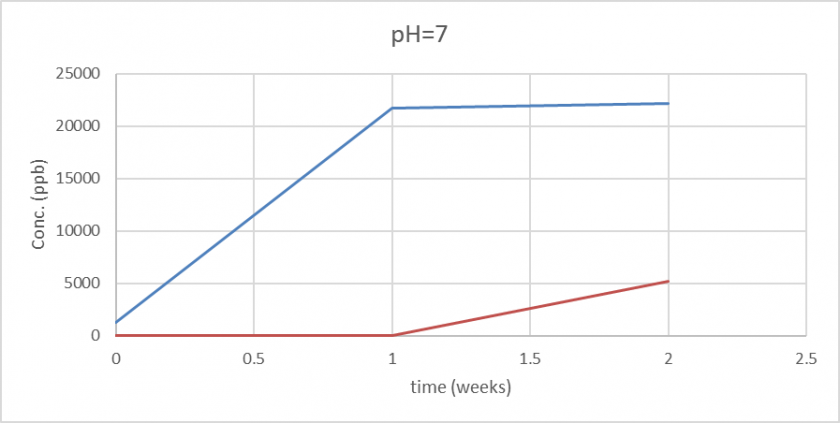
At pH 7, which is close to neutral, serpentinization reactions typically proceed at a moderate rate. The formation of serpentine minerals, such as lizardite and chrysotile, is facilitated under these conditions, with the dissolution of Mg and Fe from olivine. The ICP-MS analysis reveals that Mg is generally released in larger quantities than Fe, consistent with the fact that olivine (Mg,Fe)2SiO4 has a higher magnesium content. In contrast, the release of Fe tends to be more dependent on the specific olivine composition and the presence of other elements that may influence its solubility (Zepeda et al., 2020; Parro et al., 2017). The presence of dissolved CO2 and other trace elements can also affect the solubility of Fe, potentially leading to the formation of iron carbonates or hydroxides that precipitate out of solution (Savage et al., 2018).
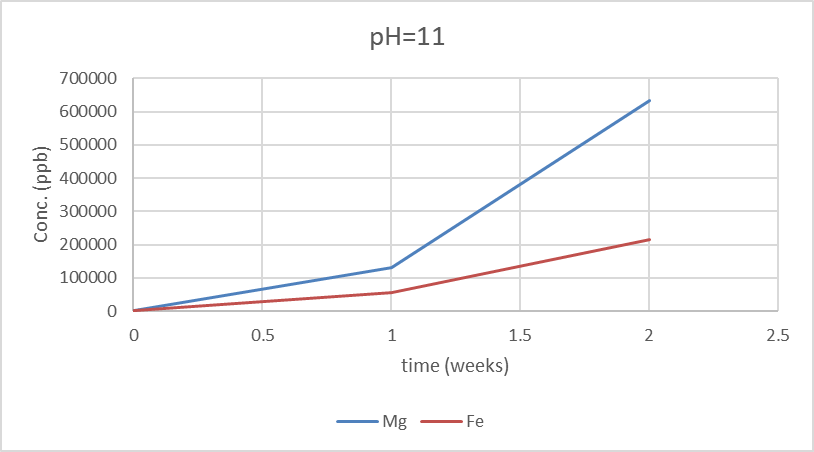
In contrast, at pH 11, which is alkaline, the serpentinization process is typically accelerated. The higher pH facilitates the dissolution of olivine and the precipitation of serpentine minerals, as the higher concentration of hydroxide ions enhances the reactivity of olivine with water. At this pH, the ICP-MS analysis shows higher concentrations of both Mg and Fe compared to the neutral pH, likely due to the more aggressive breakdown of the mineral structure. Additionally, the speciation of Fe can shift, with Fe(III) being more prevalent at higher pH, potentially forming iron oxides or hydroxides (Wang et al., 2022; Jha et al., 2021). This is in line with previous studies that have noted the impact of pH on the speciation and mobility of Fe in serpentinization reactions (Seyfried et al., 2016; McCollom & Bach, 2009).
PH 7:
PH 7 also showed an increase in concentration, but much less than the basic solution. We also can notice almost a stabilization of the concentration of magnesium after some time, reflecting the slowing of the dissolution process.