Discussion/Major Findings
We conducted autoclave reaction experiments on sandstone in deionized (DI) water under a mixture of H₂, He, and N₂. This run included the nitrogen gas reaction at 60°C and 200°C. The sandstone is an immature feldspathic sandstone, both compositionally and texturally, comprising 39% quartz, 66% feldspar, and 5% illite. Additionally, the autoclave experimental conditions included the following:
• Deionized water for a well-defined water starting composition
• 5% H2 – 2.5% He – 92.5% N2 headspace gas atmosphere; 100% N2 headspace gas for control samples
• Elevated reaction temperature of 60°C and 200°C to accelerate reaction kinetics
• Elevated pressure of 8-16 MPa to increase the partial pressure of hydrogen
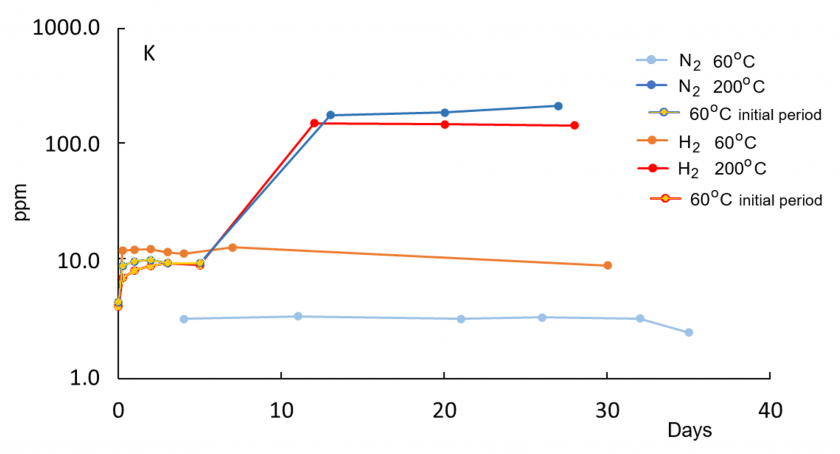
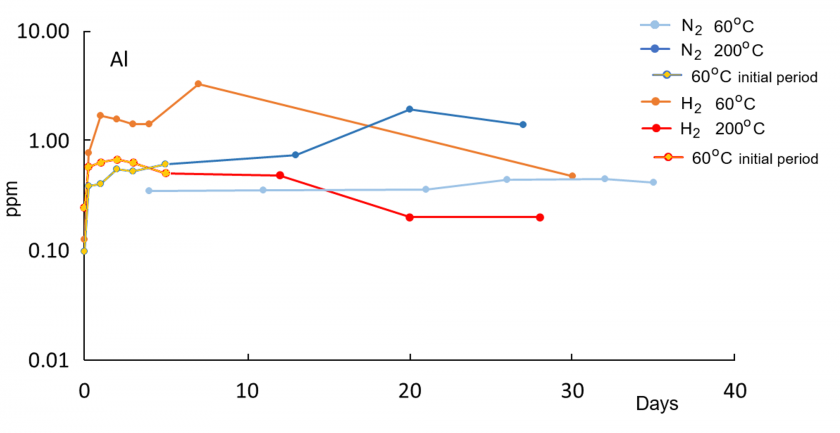
In all reaction experiments, concentrations of dissolved species increase rapidly within the first 5-6 days because of the initially low saturation of DI water. Concentrations at 200°C are generally higher compared to 60°C runs, consistent with enhanced dissolution kinetics at higher temperatures. Relative to the control run with N2 gas only, water in the H2 run at 60°C has higher concentrations of K, Al, and Fe, indicative of enhanced reactions in the presence of H2. Concentrations of Si, Na, and Ca are not elevated in the H2 solution relative to the control N2 run at 60°C. At 200°C, concentrations of K, Si, Na, and Ca are lower in the H2 run compared to the N2 run.
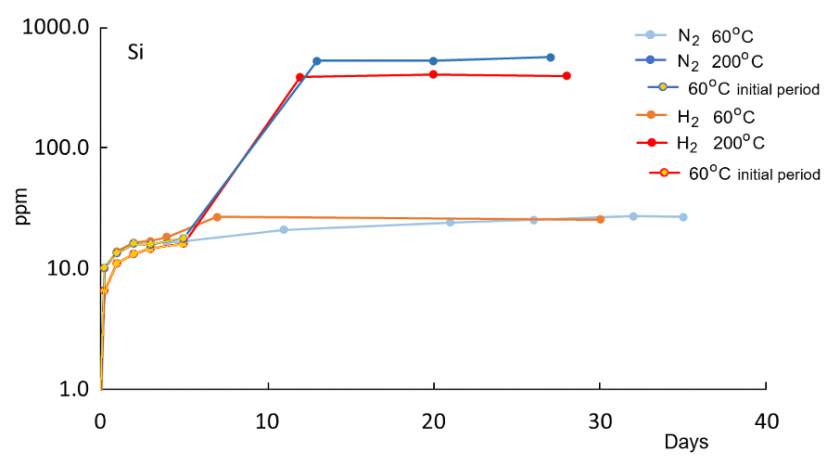
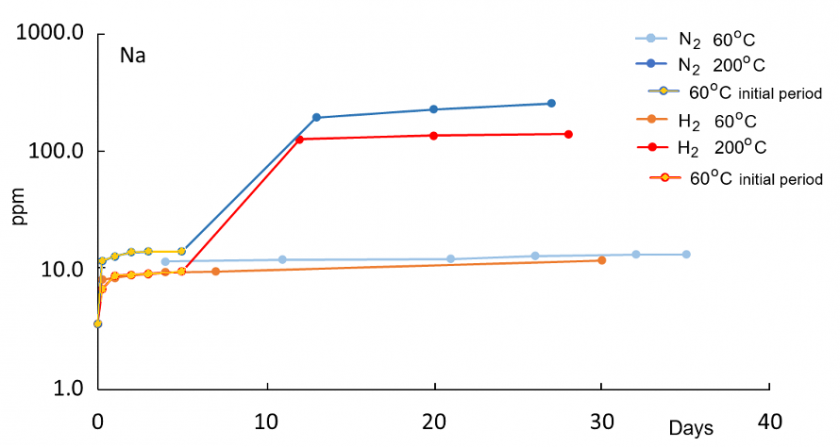
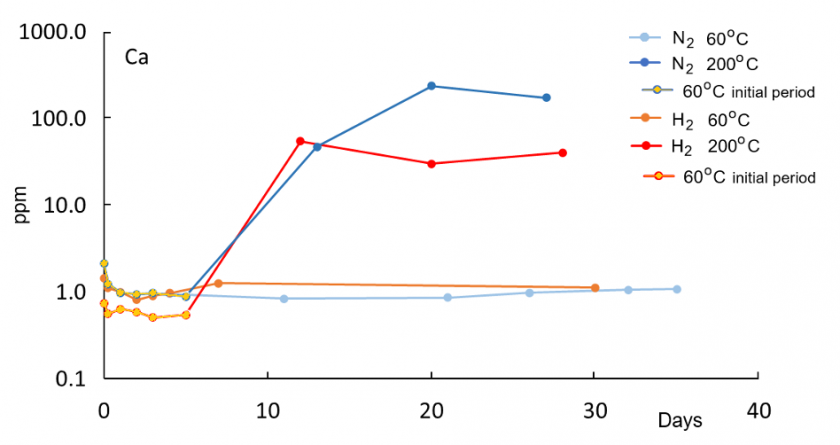
A drop in Al after an initial increase at both 60°C and 200°C indicates precipitation of newly formed solid phases. For Al, the highest concentration at 200°C in the H2 solution is lower than at 60°C, suggesting enhanced precipitation of an Al-bearing phase at 200°C. The concurrent increase in K and Al at 60°C is consistent with feldspar dissolution. The distinct later decrease in Al concentration in the H2 solution after 6-7 days at 60°C may reflect kaolinite and illite formation. All those trends are not observed in the nitrogen 60°C control run and thus appear to be enhanced in the presence of hydrogen gas.
Clay formation in a reservoir rock that might be considered for hydrogen storage can be a good indicator. Its presence can retain hydrogen and promote the layer as a good storage unit with less leaky behavior. On the other hand, excessive clay formation may destroy the reservoir’s porosity, clog its pores, and hinder it from being a hydrogen storage candidate.
Additional work, including looking for XRD and SEM images, may help confirm the precipitation of illite versus kaolinite and other solid phases in the feldspar dissolution series. Additionally, kinetic modeling for the aqueous solution reactions using modeling software such as PHREEQC might confirm the reaction and precipitation rates and whether they were consistent with water chemistry data and the potential XRD/SEM data.