Tiny Bubbles: Gardner Probes Process at Heart of Volcanic Eruptions
February 14, 2008

Volcanoes, like people, each have their own distinct personality. Hawaii’s lava oozes gently without a care in the world. Mount St. Helens and Mount Vesuvius seduce hapless strangers with their quiet slumbering beauty, then abruptly awaken in fury, crushing all in their paths. Fickle Mount Aetna can’t seem to make up its mind.
According to Jim Gardner, associate professor in the Department of Geological Sciences, the personality of a volcano has a lot to do with how easily molten rock deep underground can form bubbles. The technical term is “degassing,” a process in which gases such as carbon dioxide or water that were dissolved in the molten rock come out of solution. The National Science Foundation recently awarded Gardner a three-year, $67,000 grant to conduct a series of laboratory experiments investigating how the composition of molten rock affects degassing.
“You can imagine if it’s harder to get bubbles to nucleate, then you might be more likely to get an explosive eruption,” said Gardner. “If it’s easier, you’re going to get a much more passive eruption.”
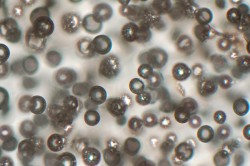
Gardner focuses on two main components of molten rock—silicate melt and gas. Silicate melt is the actual liquefied rock, made up largely of silica, as well as other elements such as iron, magnesium, sodium and potassium. The gas might be water, carbon dioxide, some other gas, or a combination.
One property of silicate melt that can affect bubble formation is surface tension—the same force that squeezes soap bubbles into spheres, rather than cubes or other more exotic shapes. In a sense, it’s a measure of how much the silicate melt molecules like to stick to each other and how much they resist bubbles elbowing in between them. The higher the surface tension, the harder it is to form bubbles and vice versa.
At the same time, certain gases or combinations of gases might have an easier or harder time degassing than others.
“With this project, we’re trying to find out where the balance is for a whole range of magma compositions—anything from basalts, which form at mid-ocean ridges or Hawaii for example, to rhyolites, like those erupting at Mount St. Helens or Yellowstone,” said Gardner. “The melt and gas composition of each location is very different.”
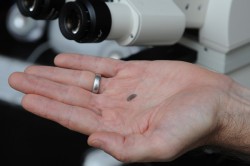
Gardner has already begun work on the project. In the Experimental Petrology Laboratory on the fifth floor of the Jackson Geological Sciences Building, he takes tube-shaped slugs of glass an inch long and about a tenth of an inch wide. Each slug is placed into a pressure chamber surrounded by a furnace. Inside, temperature and pressure are ramped up hundreds of times higher than outside. A gas is introduced and over a few days, dissolves into the molten glass. At this point, the sample is in a state somewhat akin to what real molten rock would be like far underground.

Next, the pressure is lowered to force bubble formation. Finally, the temperature is quickly dropped to freeze the bubbles in place in the solidified sample. This will help identify which set of conditions are needed to form bubbles in a particular flavor of molten rock. Using a microscope, Gardner will count the number and size of bubbles in a given volume. With an infrared spectrometer, he will measure how much gas remains dissolved in the melt.
Gardner will repeat the process many times over, varying the compositions of the rock and gas, as well as the temperature and pressure profiles. He plans to enlist graduate students to help complete the work over the next three years.
He is quick to point out that this project won’t help scientists predict if or when a particular volcano will erupt.
“It could however help us answer the question, ‘If an eruption occurs, what will it be like?’” he said.
by Marc Airhart
For more information about the Jackson School contact J.B. Bird at jbird@jsg.utexas.edu, 512-232-9623.